The Cunningham Panel™
Antibody Testing
What is the Cunningham Panel?
The Cunningham Panel™ (Autoimmune Encephalopathy and Basal Ganglia Encephalitis Panel) includes a series of high-complexity blood tests that assists clinicians in determining whether a patient’s neurologic and/or psychiatric symptoms may be due to an underlying infection-triggered autoimmune response.
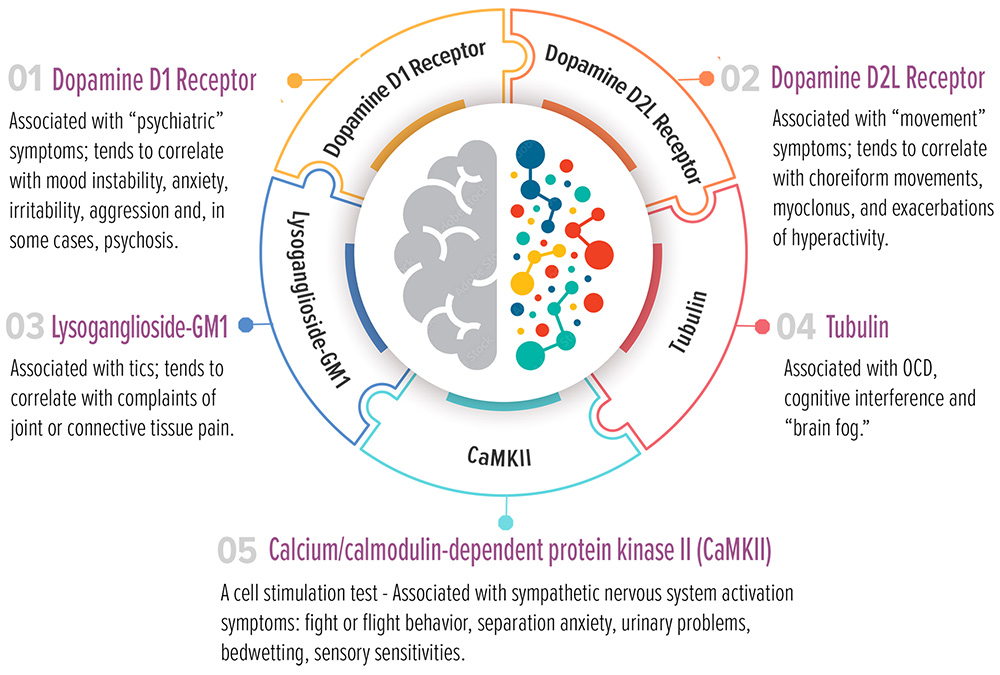
This autoimmune response may be directed against dopamine receptors, lysoganglioside, and tubulin, or it may stimulate an enzyme (CaMKII) that regulates the production of certain neurotransmitters. The Panel measures the level of autoantibodies directed against these specific targets and their ability to stimulate neurologic and/or psychiatric symptoms.
What does the test measure?
The Cunningham Panel™ includes 5 metabolic tests:
Four (4) tests measure the levels of circulating autoantibodies in serum that are directed against, and bind to or block, specific neuronal targets in the brain. Each target is associated with the presence of certain neurologic (i.e., seizures, involuntary movements) and/or psychiatric symptoms.
The fifth test measures the ability of the patient’s autoantibodies to stimulate the CaMKII enzyme that is responsible for the upregulation of brain neurotransmitters, such as dopamine, epinephrine and norepinephrine. This increase can trigger various neuropsychiatric symptoms.
Why test?
Test results can support a physician’s clinical diagnosis of autoimmune-associated seizures with laboratory evidence of an underlying autoimmune dysfunction and assist in determining an appropriate treatment regimen, such as immunomodulatory therapy.
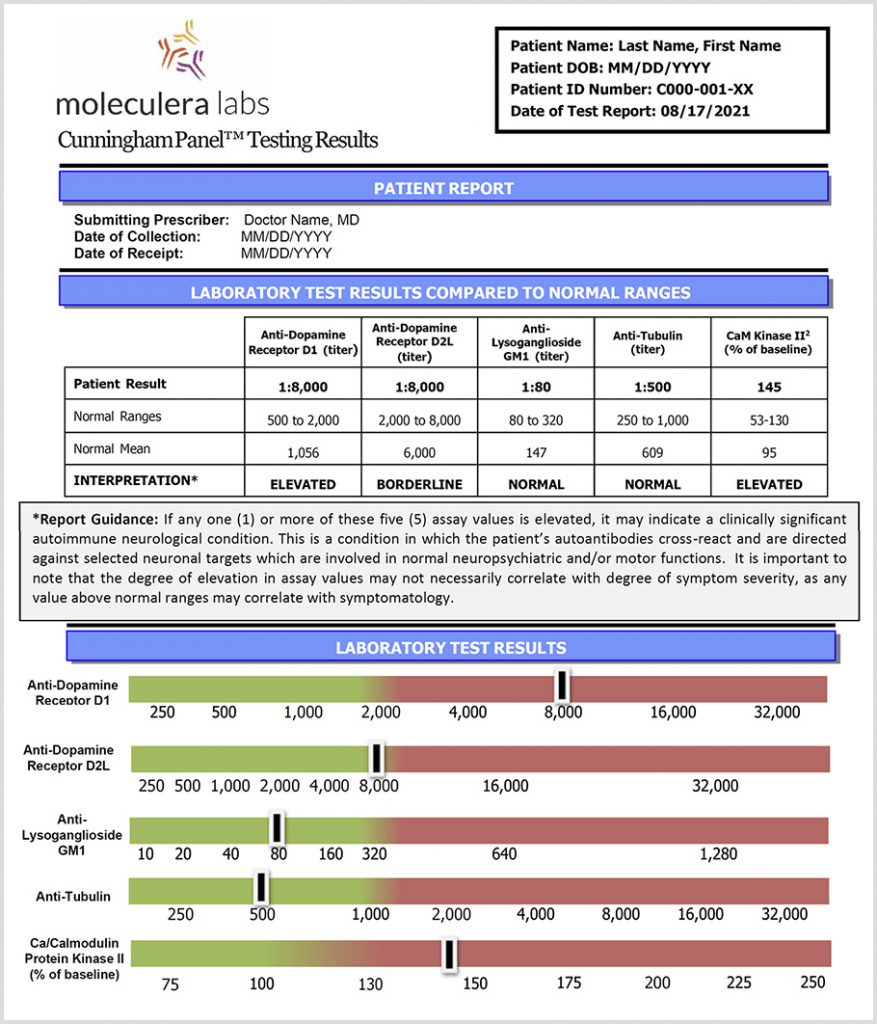
Test results are expressed as a titer, or final dilution, at which an endpoint reaction was observed on an Enzyme-Linked Immunosorbent Assay (ELISA) format.
Ordering the Cunningham Panel™ is Easy
The Cunningham Panel™ of tests requires a clinician order before we can receive and process any specimen. Moleculera Labs can accept authorized clinician test orders from all 50 states in the United States and other countries. (New York patients must have their blood collected outside the State of New York.)
Laboratory and Testing Information
Moleculera Labs is accredited by both CLIA (Clinical Laboratory Improvement Amendment) and COLA (Commission on Laboratory Accreditation) (CLIA:37D2082408; COLA:25744).